Gene therapy for hemophilia has emerged as a groundbreaking approach to transforming the lives of individuals affected by this condition. Traditionally, hemophilia patients have relied on clotting factor replacement therapies, which involve routine injections to prevent bleeding episodes. However, with recent gene therapy advances, including the FDA approval of Hemgenix, patients can now hope for a potential cure rather than a mere management solution. This revolutionary treatment reprograms the body’s own cells to produce the necessary clotting factor, reducing the burden of daily needles and allowing for a more normal lifestyle. Living with hemophilia is challenging, but these scientific innovations bring newfound optimism for those affected by hemophilia B, revolutionizing their approach to treatment and care.
The realm of gene therapy for hemophilia represents an exciting frontier in medical innovation, providing new hope to those grappling with this hereditary bleeding disorder. As alternative therapies evolve, such as the recently approved Hemgenix, many are optimistic about less invasive treatment options than conventional clotting factor therapies. Rather than mere replacements, these advanced techniques aim to genetically modify the body to produce the missing clotting factors naturally. The prospect of living without the constraints of constant needle use and frequent treatments is a major breakthrough for patients and healthcare providers alike. In this context, gene therapy is not just a treatment; it symbolizes a potential shift in how we handle hemophilia and its lifelong challenges.
Gene Therapy for Hemophilia: A Revolutionary Treatment
Gene therapy represents a significant advancement in the treatment of hemophilia, specifically hemophilia B. This condition, which is characterized by a deficiency in clotting factor IX, has long been managed through regular injections of clotting factors, which can become burdensome and impractical for patients like Terence Blue. As a groundbreaking therapy, Hemgenix offers a one-time treatment that introduces a corrected copy of the gene responsible for producing factor IX directly into the patient’s liver. This innovative approach not only reduces the need for daily or weekly injections but also promises sustained production of the clotting factor, enhancing the quality of life for those affected by hemophilia B.
The FDA’s approval of Hemgenix in November 2022 has signaled a new horizon for patients struggling with this genetic disorder. With substantial improvements in the safety and effectiveness profiles of gene therapies, the medical community is optimistic about their potential. Hemgenix allows patients to potentially eliminate the worry of spontaneous bleeding that comes with managing hemophilia. Instead of relying on frequent vital injections, patients can look forward to a future where they might manage their condition with significant less hassle, enabling a more active lifestyle without the constant fear of bleeding episodes.
Understanding Hemophilia B Treatment Options
Living with hemophilia B requires careful management and awareness of treatment options. Traditionally, patients depend on clotting factor replacement therapy, where factor IX concentrates are administered routinely to prevent bleeding episodes. However, recent advances in hemophilia B treatments, especially those involving gene therapy, have transformed care, offering patients new hope. With options like Hemgenix, which facilitates the body’s own production of necessary clotting factors, patients can now experience a significant reduction in treatment frequency, leading to a better overall quality of life.
Patients often face the daunting task of adhering to a strict treatment regimen while coping with restrictions in activities that could increase their risk of bleeding. The introduction of gene therapies such as Hemgenix aims to alleviate these burdens. Many patients express relief at the prospect of reducing their reliance on routine injections, which not only helps in managing the physical aspects of hemophilia but also addresses the emotional and social challenges of living with such a condition. Increased awareness and education among healthcare providers and patients are essential to understand these new treatment avenues and ensure optimal outcomes.
The Importance of Clotting Factor Replacement
Clotting factor replacement therapy has been the cornerstone of hemophilia treatment for decades, primarily aimed at preventing and controlling bleeding episodes in individuals diagnosed with this genetic disorder. Patients like Terence Blue have traditionally required frequent injections of synthetic or plasma-derived clotting factors, a regimen tedious yet crucial for managing their health. This therapy is particularly vital for those with severe hemophilia, where spontaneous bleeding can lead to severe complications and long-term joint damage.
However, advancements in hemophilia management are shifting the landscape from simple replacement therapies to more innovative solutions. Today, emerging therapies are not only improving safety and efficacy profiles but are also paving the way for potential cures through gene therapy. This evolution is essential in extending a normal lifespan and enhancing the quality of life for those living with hemophilia, minimizing the burden associated with continuous treatment protocols.
The Future of Hemophilia Treatments: Clinical Trials and Research
The future of hemophilia treatments looks increasingly promising due to the ongoing research and clinical trials exploring a variety of therapeutic approaches. Late-breaking studies have shown great potential in gene therapy as a long-term solution for hemophilia B, providing safer, and potentially curative options. As we saw with Hemgenix, many patients show a significant response leading to stable levels of factor IX production, lessening the need for conventional factor replacement therapy.
Moreover, the expansion of clinical trials to include various demographics and severity levels of hemophilia is crucial. The data generated from these trials will enhance our understanding of gene therapy’s long-term efficacy in diverse populations. This focus on evidence-based approaches is necessary to ensure that both patients and healthcare providers can make informed decisions about treatment plans, nourishing hope for better management and potential cures.
Living with Hemophilia: A Personal Journey
Living with hemophilia is more than just about managing physical health; it involves navigating emotional and social challenges that arise from the condition. Individuals like Terence Blue illustrate the profound impact that hemophilia has not only on their physical abilities but also on their lifestyle choices and relationships. The constant need for self-care and the anxiety associated with potential bleeding episodes can lead to isolation, as social activities sometimes fall by the wayside due to the fear of injury.
However, new treatments, especially gene therapies such as Hemgenix, offer hope for a brighter future. By significantly reducing the risk of spontaneous bleeds and the need for daily injections, these treatments empower patients, allowing them to reclaim aspects of their lives that were previously hindered by their condition. Overcoming social stigmas, enjoying physical activities, and fostering relationships without the constant burden of hemophilia allows individuals to redefine what living with the condition truly means.
Economic Challenges of Gene Therapy in Hemophilia
Despite the monumental advancements in gene therapy for conditions like hemophilia, economic factors play a critical role in ensuring that these treatments are accessible to patients. The high cost of therapies, such as Hemgenix, which can exceed millions of dollars, raises significant concerns regarding insurance coverage and patient affordability. While these therapies demonstrate incredible potential for long-term health benefits, their price points may limit accessibility for many patients across different healthcare systems.
Insurance companies may be hesitant to cover the cost of high-priced gene therapies, particularly if there is uncertainty about long-term outcomes and effectiveness. This creates a challenging landscape where patients must advocate for access to treatments that could profoundly improve their lives. Hence, ongoing dialogue between healthcare providers, policymakers, and patients is essential to navigate the economic hurdles associated with these advanced therapies, ensuring equitable access to innovative treatments for hemophilia.
Regulatory Landscape for Hemophilia Treatments
The regulatory landscape governing gene therapies for hemophilia is continuously evolving as more treatments emerge and physicians begin integrating them into clinical practice. The approval of Hemgenix by the FDA serves as a significant milestone, yet it also highlights the complexities of validating new therapies. This includes rigorous evaluation processes that assess efficacy and safety, as well as the economic viability of these high-cost interventions for broader patient populations.
Regulatory bodies are tasked with striking a balance between fostering innovation in hemophilia treatments and ensuring patient safety. Deliberations around pricing, coverage, and reimbursement must also align with the realities faced by patients and healthcare systems. The interaction between regulatory approvals and market dynamics is crucial in determining which therapies become widely available, underscoring the importance of collaboration among researchers, regulatory agencies, and healthcare providers.
Patient Experiences: Testimonials and Insights
Patient experiences provide invaluable insights into the impact of hemophilia treatments, particularly as new innovations like gene therapy gain traction. Individuals like Terence Blue are bridging the gap between clinical efficacy and real-world applications, sharing their journeys to highlight both the challenges and successes encountered along the way. Their testimonials underscore the profound transformations that can arise from effective treatment, showcasing stories of resilience and hope.
Moreover, testimonials help demystify these advanced therapies, shedding light on the patient mindset regarding gene therapy. As stories circulate about the positive experiences and challenges faced by individuals undergoing treatments like Hemgenix, the community grows more informed, creating a supportive environment that encourages dialogue about treatment options. This peer support is essential for fostering a sense of community among those living with hemophilia, reminding them that they are not alone in their journeys.
Challenges in Accessing New Hemophilia Therapies
As the field of hemophilia treatment evolves, patients often face numerous challenges in accessing innovative therapies. Safety and quality are paramount, but even once therapies like Hemgenix gain approval, the real test often lies in their availability to those in need. Navigating healthcare systems, insurance policies, and regional disparities can create barriers that prevent many patients from receiving potentially life-changing treatments.
Additionally, the emotional toll of waiting for new treatments to reach the market cannot be underestimated. Patients and their families maintain hope amid uncertainty, eagerly anticipating the day when approved therapies become broadly accessible. Ongoing advocacy efforts aim to address these issues, emphasizing the need for equitable access to cutting-edge treatments, regardless of economic or geographic factors. The collective push for improved access to therapeutic options ensures that the promise of hemophilia gene therapy can be fulfilled for all patients.
Frequently Asked Questions
What is gene therapy for hemophilia and how does it work?
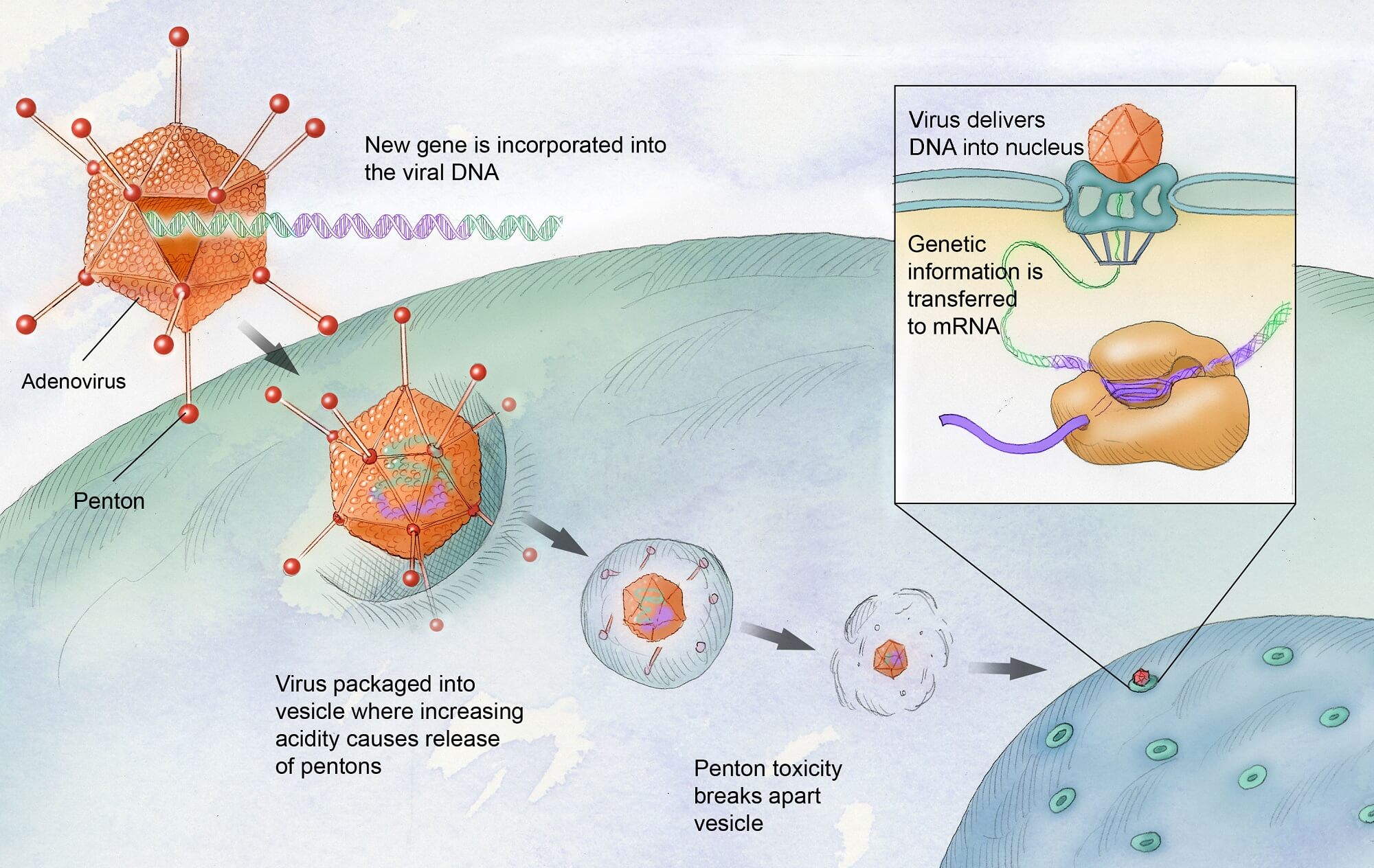
Gene therapy for hemophilia is a revolutionary treatment method that aims to correct genetic defects responsible for the disease, particularly in hemophilia B. This approach utilizes a viral vector to deliver a healthy copy of the gene that produces clotting factor IX directly into the liver, enabling the body to produce this crucial protein naturally and reducing or eliminating the need for routine clotting factor replacement.
What are the latest gene therapy advances for hemophilia B treatment?
Recent advances in gene therapy for hemophilia B include the development of Hemgenix, which received FDA approval in November 2022. This therapy has shown promising results, allowing patients to potentially overcome the limitations of traditional treatments, such as frequent clotting factor injections.
How has Hemgenix changed the landscape of hemophilia B treatment?
Hemgenix marks a significant breakthrough in hemophilia B treatment by providing a one-time gene therapy that can potentially eliminate the need for daily or frequent clotting factor replacements. Early reports indicate that patients receiving this therapy may sustain normal levels of clotting factor IX, enhancing their quality of life and reducing the burden of living with hemophilia.
What challenges do patients face when considering gene therapy for hemophilia?
Patients considering gene therapy for hemophilia face several challenges, including high treatment costs, potential side effects, and the need for thorough evaluation and monitoring. Additionally, the therapy’s high price can lead to insurance hurdles, making it vital for patients to have open discussions with healthcare providers about the financial and medical aspects before proceeding.
How does living with hemophilia change after receiving gene therapy?
After receiving gene therapy for hemophilia, many patients experience significant lifestyle improvements, including reduced anxiety about bleeding episodes and the elimination of daily injections. Patients like Terence Blue have reported faster healing times and an increased sense of normalcy in their daily lives, although long-term efficacy and monitoring are essential to ensure sustained improvements.
What is the impact of Hemgenix on the future of hemophilia treatment?
The impact of Hemgenix on the future of hemophilia treatment is profound, as it represents a shift towards curative options rather than lifelong management through prophylactic therapies. With data showing a majority of patients not requiring factor replacement three years post-treatment, this therapy provides hope for permanent solutions and may encourage further research in genetic treatments for other conditions.
What are the potential side effects of gene therapy for hemophilia?
Potential side effects of gene therapy for hemophilia can include liver enzyme elevations, allergic reactions, and the risk of immune responses to the viral vector used for gene delivery. Close monitoring by healthcare professionals during and after therapy is crucial to manage these risks effectively.
How does gene therapy for hemophilia differ from traditional clotting factor replacement?
Gene therapy for hemophilia differs from traditional clotting factor replacement by targeting the underlying genetic cause of the disease. While clotting factor replacements require ongoing administration to manage symptoms, gene therapy aims to provide a long-term solution by correcting the genetic defect and allowing the body to produce clotting factors naturally.
What should patients expect regarding the cost of gene therapy for hemophilia?
Patients can expect the cost of gene therapy for hemophilia to be significant, with treatments like Hemgenix priced at approximately $3.5 million. However, insurance coverage and negotiations may lower these costs. Patients should consult with their healthcare providers and financial advisors to understand the implications for their treatment options.
Can gene therapy for hemophilia eliminate the need for future treatment?
Gene therapy for hemophilia has the potential to eliminate or drastically reduce the need for future treatment, particularly in cases like hemophilia B. Early clinical trials have shown that many patients who received Hemgenix do not require further factor replacement after treatment, suggesting a long-lasting benefit.
| Key Point | Details |
|---|---|
| Terence Blue’s Journey | Terence Blue, diagnosed with hemophilia B early in life, has undergone significant treatment and finally received gene therapy. |
| Gene Therapy Introduction | In February 2025, Blue became the first patient in New England to receive Hemgenix, a new gene therapy approved by the FDA. |
| Mechanism of Action | Hemgenix works by using a virus to deliver corrected genes to the liver, where they produce clotting factor IX. |
| Market Challenges | Despite potential, gene therapies face market pressure and high costs; Hemgenix is priced at $3.5 million. |
| Positive Outcomes | As of mid-March, Blue’s factor IX levels improved significantly, indicating the therapy’s success. |
| Community Impact | The development of gene therapy promises to change the lives of hemophilia patients, reducing reliance on traditional treatments. |
Summary
Gene therapy for hemophilia represents a groundbreaking advancement in the treatment of this condition. Terence Blue’s successful experience with Hemgenix illustrates the potential for gene therapy to change lives by providing lasting relief from traditional treatment methods, ultimately allowing patients to live more freely and without constant worry.