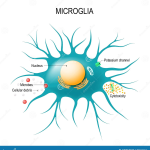
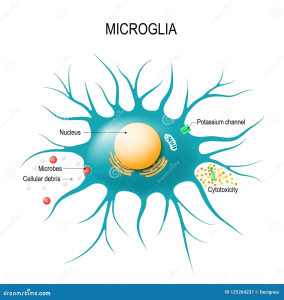
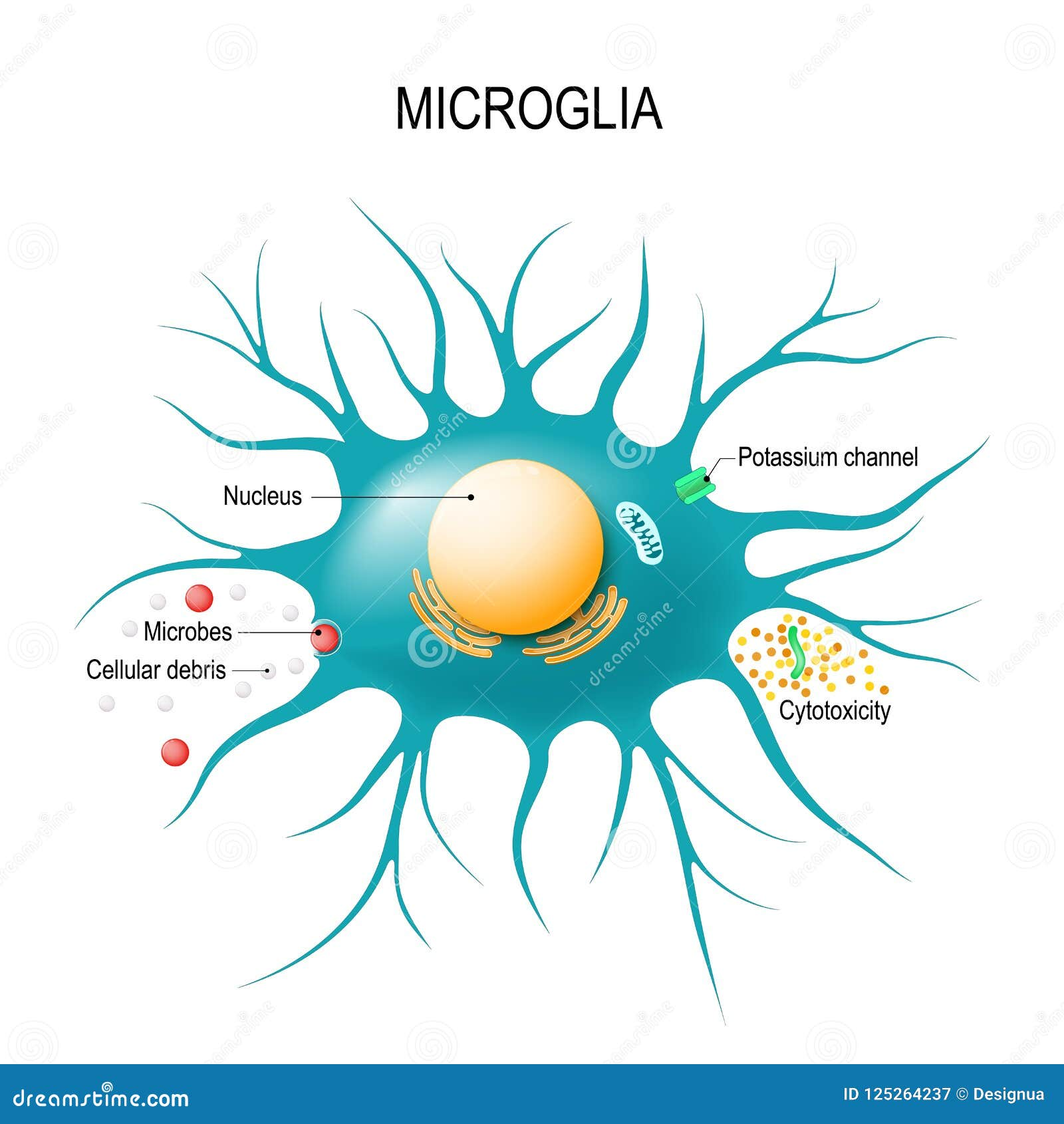
Microglial cells are essential components of the brain’s immune system, playing a crucial role in maintaining brain health. As the first responders to injury and disease, these fascinating cells patrol the central nervous system, seeking out damaged neurons and debris, a process that has significant implications for neurodegenerative diseases like Alzheimer’s disease. Recent neuroscience research has revealed that abnormal activity of microglia can exacerbate conditions such as Alzheimer’s by failing to efficiently clear away cellular waste. This critical discovery has opened new avenues for identifying biomarkers for Alzheimer’s, offering hope for earlier diagnosis and targeted therapies. By understanding microglial function, researchers aim to reshape our approach to brain health and potentially revolutionize treatment options for millions affected by neurodegenerative disorders.
Also referred to as brain macrophages, microglial cells serve a vital role in the neurological landscape, functioning as the guardians of the central nervous system. Their primary responsibility involves monitoring neuronal health and responding to potential threats, which ties closely to the pathology of various brain diseases. Studies have shown that these immune cells can significantly influence the progression of neurodegenerative conditions, including Alzheimer’s, by their involvement in synaptic pruning and inflammation. The ongoing exploration of these unique immune cells highlights a pivotal area in contemporary neuroscience, enriching our understanding of brain function and its associated pathologies. As the search for effective treatments continues, microglial cells might emerge as key players in the quest for remedies against debilitating neurodegenerative diseases.
Understanding Microglial Cells in Alzheimer’s Disease
Microglial cells, often referred to as the brain’s immune system, play a pivotal role in maintaining brain health. These cells constantly monitor the central nervous system, ready to respond to cellular debris, infections, and injuries. However, in the context of Alzheimer’s disease, their function can become dysregulated, leading to increased inflammation and neurodegeneration. Recent neuroscientific research has illustrated a connection between the abnormal activities of microglia and the development of neurodegenerative diseases like Alzheimer’s and Huntington’s disease.
The interplay between microglia and neuronal health is crucial. When functioning properly, microglia assist in the remodeling of synapses, which are key to neural communication. Yet, when these cells overreact or engage in excessive synaptic pruning, they contribute to the pathophysiology of Alzheimer’s disease. Investigations into this phenomenon are vital as they not only deepen our understanding of fundamental brain processes but also pave the way for identifying new biomarkers for Alzheimer’s. By targeting microglial activity, researchers hope to unveil innovative treatment avenues that could mitigate the impacts of this devastating disease.
The Role of Synaptic Pruning in Neurodegenerative Diseases
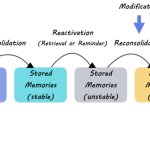
Synaptic pruning is a natural process that refines neural circuits during development and learning. Yet, in neurodegenerative diseases like Alzheimer’s, this mechanism can spiral out of control, leading to excessive loss of synapses and neuronal connections. Findings from Beth Stevens’ lab have drawn attention to how microglial cells can misinterpret signals in the brain, resulting in aberrant pruning that exacerbates the early signs of Alzheimer’s disease. By targeting the pathways involved in these processes, scientists can gain critical insights into how to preserve brain health as we age.
Researchers are now focusing on developing therapeutic strategies to regulate microglial activity and correct abnormal synaptic pruning. The hope is that by understanding the triggers that cause microglia to erringly begin pruning excess synapses, new treatments can be developed to restore normal function and protect against neurodegeneration. Making advancements in this area could significantly impact how we approach Alzheimer’s disease and other related disorders, leading to better care and improved quality of life for millions affected by these conditions.
Neuroscience Research and Its Impact on Alzheimer’s Treatment
The field of neuroscience has seen rapid advancements in understanding the cellular mechanisms behind Alzheimer’s disease. As highlighted by Beth Stevens’ pioneering research, investigating the roles of microglia and synapses is key to unlocking new diagnostic and therapeutic options. Research initiatives that focus on the basic science underpinning these cellular interactions provide the groundwork necessary for the development of novel treatments. This knowledge is steering us toward potential breakthroughs that could transform Alzheimer’s care.
With an estimated 7 million Americans living with Alzheimer’s, there is an urgent need for effective therapies and preventive strategies. Neuroscience research not only seeks to unravel the complexities of neurodegenerative diseases but also strives to translate these findings into practical applications. As funding from the National Institutes of Health (NIH) continues to support these efforts, the potential for discovering impactful biomarkers holds promise for early detection and intervention in Alzheimer’s disease, ultimately shifting the focus from treatment to prevention.
Funding and Support for Alzheimer’s Research
The journey of Alzheimer’s research is heavily reliant on federal funding and grants. Beth Stevens emphasized the importance of continuous support from the NIH, which has enabled her lab to push the boundaries of our understanding of neurodegenerative diseases. The foundation provided by such funding not only facilitates innovative studies into microglial function but also enables scientists to translate their findings into real-world impacts, such as the development of therapeutic options for Alzheimer’s disease.
However, the challenge remains that basic science can often seem removed from direct medical applications, leading to questions regarding the relevance of such research. Yet, as demonstrated in Stevens’ work, studying seemingly distant processes in animal models often leads to pivotal discoveries that can inform human health interventions. As we invest in the future of neuroscience research, the outcomes promise to enrich our understanding of brain diseases and provide hope for those at risk of Alzheimer’s.
Emerging Biomarkers for Alzheimer’s Disease
In the quest to combat Alzheimer’s disease, identifying reliable biomarkers has become a focal point. Biomarkers have crucial implications for diagnosing Alzheimer’s early and accurately, ultimately facilitating timely interventions. Beth Stevens’ research showcases how anomalies in microglial activity may serve as a precursor to the disease, offering a potential avenue for biomarker development. These insights allow for better monitoring of disease progression and can guide future therapeutic strategies.
The search for biomarkers is driving innovative approaches that integrate genetic, biochemical, and imaging technologies. Identifying early indicators of Alzheimer’s can significantly alter patient management and enhance therapeutic outcomes. As research continues to unfold, the hope is to establish a panel of biomarkers that not only aids in early detection but also predicts disease trajectory, thereby transforming Alzheimer’s blindness into an informed strategy for treatment and support for those affected.
The Importance of Basic Science in Neuroscience Research
Understanding the roots of Alzheimer’s disease hinges on basic scientific research. As scientists delve into the mechanisms that govern microglial function, synaptic pruning, and neuronal health, they uncover essential features that inform clinical practice. Basic science endeavors often lay the groundwork for groundbreaking discoveries. Beth Stevens’ experience exemplifies how curiosity-driven research has illuminated previously uncharted territories within neuroscience, ultimately guiding new treatment paradigms for Alzheimer’s.
Investing in basic science programs also fosters a culture of inquiry that can lead to unexpected findings with significant clinical implications. By prioritizing exploration of the brain’s immune system and its effects on neurodegeneration, researchers can make strides toward understanding complex brain disorders. The intertwining of fundamental research with translational science is essential; it connects laboratory discoveries to real-world applications, benefitting individuals affected by neurodegenerative diseases.
Microglia: Guardians of Brain Health
Microglial cells are essential for maintaining brain health, acting as the first line of defense against neurological threats. Their role includes not only surveillance and response to damage but also the intricate process of pruning synapses to ensure proper brain function. In the context of Alzheimer’s disease, understanding how these cells navigate their tasks becomes crucial. Dysregulation in microglial responses can lead to increased inflammation, contributing to the onset and progression of neurodegenerative diseases.
Recent advancements in neuroscience research are shedding light on potential therapeutic interventions that target microglial function. By harnessing the power of these immune cells, researchers aim to develop strategies that could quell neuroinflammation and promote neuronal survival. This line of inquiry reinforces the significance of microglial health in preserving cognitive function and underscores the necessity of rigorous study into their biology, especially in relation to Alzheimer’s disease.
Neuroprotection and Alzheimer’s Disease
Neuroprotection is a critical consideration in the fight against Alzheimer’s disease. The mechanisms that lead to neuronal death often intertwine with the activities of microglial cells, whose overzealous response can result in synaptic loss. Understanding these relationships is vital for developing treatment strategies aimed at protecting neurons from the neurodegenerative processes common in Alzheimer’s. Research is increasingly focusing on how to enhance the neuroprotective roles of microglia while mitigating their destructive potential.
Recent studies suggest that directing microglial activity toward beneficial pathways can rebuild neural connections and foster a healthier brain environment. Therapeutic agents that modulate microglial behavior represent a promising direction in Alzheimer’s research. By taking a proactive approach to neuroprotection, scientists hope to slow disease progression and improve outcomes for those affected by Alzheimer’s, ultimately transforming how we manage neurodegenerative diseases at large.
The Future of Alzheimer’s Research and Treatment
As we look to the future of Alzheimer’s research and treatment, the integration of innovative ideas is paramount. The current understanding of the roles of microglial cells and synaptic pruning is just the beginning of a long journey toward effective intervention strategies. Ongoing studies are focusing on how we can harness these biological processes for therapeutic gain, including the development of targeted drugs that could amend the aberrant behavior of microglia in Alzheimer’s patients.
The prospect of developing effective Alzheimer’s treatments is not only a scientific challenge but also a moral imperative. With the number of individuals living with Alzheimer’s set to increase dramatically, there is an urgent need for actionable solutions. By capitalizing on current biomedical research and fostering a collaborative environment among scientists, clinicians, and funding agencies, the hope is to unveil groundbreaking therapies that could reshape the landscape of Alzheimer’s disease management.
Frequently Asked Questions
What are the roles of microglial cells in Alzheimer’s disease?
Microglial cells function as the brain’s immune system, playing a crucial role in Alzheimer’s disease by monitoring for injury and clearing dead cells. However, in Alzheimer’s, these cells can become overactive, leading to inappropriate synaptic pruning, which contributes to neurodegeneration. Therefore, understanding microglial cell behavior is essential for developing new therapies and biomarkers for Alzheimer’s.
How do microglial cells influence brain health in neurodegenerative diseases?
Microglial cells are vital to maintaining brain health as they respond to cellular changes and clear debris. In neurodegenerative diseases like Alzheimer’s, their dysregulation can exacerbate neural damage and inflammation. Research into microglial functions offers insights into potential interventions that could protect brain health and slow disease progression.
What is the significance of microglial cells in neuroscience research?
In neuroscience research, microglial cells are significant because they reveal how the immune system interacts with the brain’s environment. Studies have shown that abnormalities in microglial activity are linked to neurodegenerative diseases, particularly Alzheimer’s. This understanding is pivotal for developing novel strategies for diagnosis and treatment.
Can microglial cells serve as biomarkers for Alzheimer’s disease?
Yes, microglial cells can serve as biomarkers for Alzheimer’s disease. Research is ongoing to identify specific changes in microglial activity or markers that correlate with disease progression. By proving reliable indicators of Alzheimer’s, these biomarkers could enhance early diagnosis and track treatment efficacy.
What discoveries about microglial cells have impacted the treatment of Alzheimer’s disease?
Recent discoveries about microglial cells have reshaped our approach to treating Alzheimer’s disease. Insights into their role in synaptic pruning and inflammation provide new avenues for targeted therapies that could mitigate neurodegenerative processes and improve patient outcomes.
How do microglial cells contribute to synaptic pruning during brain development?
During brain development, microglial cells are essential for synaptic pruning, which is the process of removing excess synapses to fine-tune neural circuits. This normal function of microglia ensures that the brain operates efficiently; however, when this process becomes dysregulated, it can lead to neurodegenerative diseases such as Alzheimer’s.
What is the relationship between microglial cells and neuroinflammation in neurodegenerative diseases?
Microglial cells are central to neuroinflammation, acting as immune responders in the brain. In conditions like Alzheimer’s disease, chronic activation of microglia can lead to sustained inflammation, contributing to neuronal damage and disease progression. Understanding this relationship is key to developing anti-inflammatory treatments.
What ongoing research is being done on microglial cells and Alzheimer’s disease?
Ongoing research focuses on understanding the complex roles of microglial cells in Alzheimer’s disease, including their neuroprotective and neurotoxic functions. Scientists are exploring how to modulate microglial activity to enhance brain health and slow disease progression, paving the way for innovative therapies and preventive strategies.
| Key Points |
|---|
| Microglial Cells as Immune System |
| Microglial cells patrol the brain for illness and injury, aiding in the clearance of dead cells and synapse pruning. |
| Impact on Neurodegenerative Diseases |
| Aberrant pruning by microglia contributes to diseases like Alzheimer’s and Huntington’s, leading to potential new treatments. |
| Basic Science Research Importance |
| Curiosity-driven research in microglial cells has paved the way for understanding and treating neurological disorders. |
| Role of NIH Funding |
| Stevens emphasizes that NIH and federal funding have been crucial for foundational research, driving discoveries in the field. |
Summary
Microglial cells play a crucial role in protecting the brain and are key players in the immune response within the central nervous system. Understanding the complexities of microglial function, particularly in processes like synaptic pruning, has led to significant insights into neurodegenerative diseases such as Alzheimer’s. The groundbreaking work of researchers like Beth Stevens highlights how basic scientific inquiry can unearth essential mechanisms that, while initially obscure, hold immense potential for developing new diagnostic biomarkers and therapeutics. As research continues, the link between microglial dysfunction and neurological disorders becomes increasingly relevant, underscoring the importance of funding and support for scientific discovery in this vital realm.