Medical research funding plays a critical role in advancing healthcare and enhancing patient safety across the globe. However, recent NIH funding cuts have cast a dark shadow over ongoing studies, jeopardizing both the integrity of research and the well-being of participants. As critical oversight mechanisms like Institutional Review Board (IRB) oversight become compromised, concerns arise about the ethical conduct of clinical trials. With funding cuts halting vital projects, the impact of funding on research cannot be overstated; it affects everything from recruitment strategies to data monitoring practices, ultimately influencing patient outcomes. As we navigate these turbulent waters, the ethical implications of funding on research practices become a focal point in discussions surrounding clinical research ethics.
Investing in biomedical exploration and innovation is paramount for enhancing medical care and ensuring participant protection in various trials. The reduction of governmental financial support, especially from agencies like the NIH, undermines essential services and could lead to grave consequences for patient safety in research initiatives. Compromised funding structures stir questions about the ethical frameworks that govern clinical investigations, as the collaboration between research institutions, funding bodies, and oversight groups becomes strained. Such a scenario highlights the pressing need for sustainable funding strategies that can bolster the integrity of investigations while protecting the rights and welfare of individuals who volunteer for research. Ultimately, fostering a healthy ecosystem for research financing ensures the continuous evolution of healthcare solutions and safeguards public trust in the research process.
The Impact of NIH Funding Cuts on Patient Safety
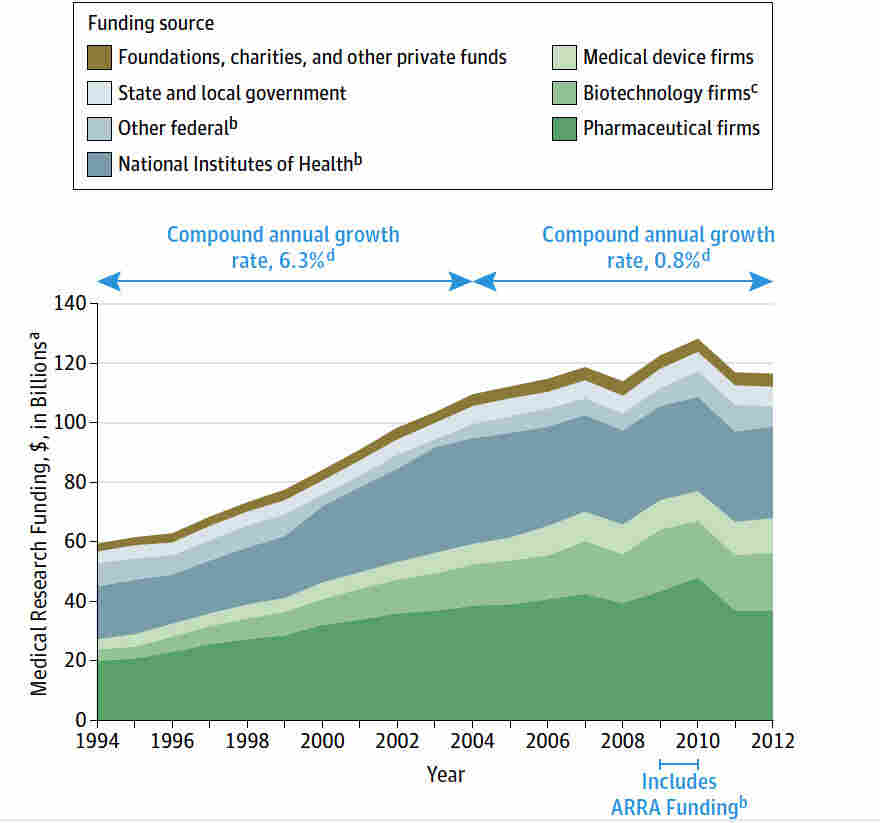
The recent NIH funding cuts have raised alarms regarding the safety and rights of patients involved in medical research. These cuts, totaling over $2 billion, significantly hinder the ability of institutions like Harvard to conduct thorough Institutional Review Board (IRB) oversight. The IRBs play a crucial role in safeguarding participants by reviewing study protocols to ensure ethical standards are met, which ultimately protects patient interests. Without sufficient funding, the capacity of IRBs to conduct extensive reviews is jeopardized, potentially exposing participants to unregulated research practices.
Reduced funding impacts not only the operational aspects of IRBs but also their capacity to engage in continual education and oversight training for investigators. Patient safety is inherently connected to the rigor of ethical review processes; thus, when funding sources dwindle, the likelihood of maintaining high standards of participant protection diminishes. Ultimately, funding cuts directly correlate to an increased risk of safety lapses, which harms both individuals and the integrity of research outcomes.
The Role of IRB Oversight in Clinical Trials
Institutional Review Boards (IRBs) serve as vital gatekeepers in clinical research, tasked with the responsibility of protecting patient rights and safety. They are responsible for ensuring that proposed research meets ethical standards while addressing potential risks related to study participation. This oversight is especially crucial in a landscape where research often involves vulnerable populations, and the need for transparency is paramount. Effective IRB operations rely on collaborative funding initiatives, which enable regular reviews and updates to protocols that prioritize patient welfare.
Despite their importance, IRB processes can be time-consuming and resource-intensive. Funding cuts may cause delays in the review and approval of studies, ultimately slowing the advancement of critical medical research. As research institutions grapple with financial strain, the necessary scrutiny that IRBs provide can wane, leading to adverse consequences for patient safety. Therefore, the connection between robust IRB oversight and the ethical conduct of clinical trials underscores the critical need for sustained funding in the field of medical research.
Ethical Dimensions of Clinical Research During Funding Cuts
The ethical implications of medical research funding cuts extend beyond just financial constraints; they touch upon the trust that the public places in research institutions. When funding dries up, it can lead to compromised research integrity, where ethical considerations may be sidelined due to budget pressures. This creates a moral dilemma for researchers who are obligated to prioritize patient safety while navigating the possible repercussions of halted studies or lost collaborations. Without adequate resources, researchers may find it challenging to uphold the ethical obligations enshrined in the IRB approval process.
Moreover, the historical context of medical research ethics plays a pivotal role in understanding the consequences of funding cuts. Past transgressions highlight the importance of strict ethical oversight to prevent reoccurrence of violations such as those seen in the Tuskegee Syphilis Experiment. Today, the systematic review of studies by IRBs is designed to prevent these issues, but without financial backing, the infrastructure for ethical compliance can weaken. This underscores the critical link between funding and ethical mandates, emphasizing the need for a well-resourced IRB system to maintain public trust in the medical research process.
Assessing the Broader Impact of Research Funding Cessation
The cessation of research funding has wide-ranging consequences that extend beyond immediate patient safety concerns to encompass broader societal health implications. For example, when large grants are suspended, entire research programs may be forced to shut down, resulting in lost opportunities for groundbreaking medical discoveries. This can also hinder the development of new treatments for chronic diseases, such as Alzheimer’s, which require extensive collaboration across various institutions. The cumulative effect of these funding cuts can lead to stagnation in medical advancement, with serious repercussions for public health.
Furthermore, the loss of funding can exacerbate mistrust in the medical research community. Communities that have historically been wary of clinical trials due to past ethical abuses may see funding cuts as evidence of ongoing disregard for participant safety. This creates a vicious cycle where reduced engagement from communities leads to fewer participants in vital studies, ultimately undermining the diversity and relevance of clinical research findings. As we consider the implications of funding cuts, it becomes clear that sustained financial support is essential to preserve both the integrity of the research process and the trust of the public.
Navigating Patient Concerns in a Funding Crisis
In the wake of funding cuts, patient concerns regarding their safety and the ethical conduct of clinical trials become increasingly pronounced. Patients who voluntarily participate in research studies rightfully expect that their rights and welfare will be prioritized. However, with the limitations imposed by reduced funding, research institutions may struggle to maintain the same level of oversight and support as before. This scenario places patients in a vulnerable position, raising questions about their informed consent and the level of risk they may be unwittingly accepting.
It’s crucial for institutions to address these concerns proactively, ensuring transparency in how funding disruptions may affect ongoing research. Continued communication with patients can help mitigate fears and reinforce their importance in the research process. Research institutions must demonstrate their commitment to patient safety by exploring alternative funding sources or prioritizing essential oversight functions in their budgets to uphold their ethical obligations and protect the participants who form the backbone of clinical research.
Enhancing Public Trust Through Transparency
In light of funding cuts, fostering public trust in medical research becomes more challenging yet essential. Transparency about the impacts of funding disruptions on clinical trials can help reassure participants that their safety remains a priority. Institutions should communicate openly about their commitment to ethical research practices, even amid financial uncertainty. By actively engaging with communities, institutions can reinforce their dedication to patient rights, thereby alleviating concerns and cultivating a more trusting relationship.
Publicly sharing information about the steps being taken to mitigate funding impacts—such as training IRB members, adjusting oversight protocols, or seeking additional partnerships—can also foster a sense of shared responsibility. Institutions can engage in community forums or outreach programs that allow for feedback and discussion on the ethical implications of research practices. By embracing a culture of transparency, medical research institutions can strengthen public trust and ensure continued support for clinical trials, even in a funding-challenged environment.
Long-Term Effects of Disruption in Medical Research
The long-term effects of disruption in medical research funding can be profound, impacting not only current studies but also the future landscape of medical advancements. As funding becomes scarce, researchers may opt to focus on shorter-term studies with immediate outputs to secure limited resources. This shift can stifle innovation and limit the exploration of essential but complex research topics that require sustained investment. Consequently, vital research areas may languish, depriving patients of new treatments and therapies that can significantly improve their quality of life.
Moreover, the adverse consequences on research ethics cannot be overlooked. When funding limitations lead to hurried ethics reviews or inadequate oversight, the potential for unethical practices increases. Such lapses carry the risk of undermining decades of progress in patient rights and safety, setting back the gains made in public trust and ethical standards in medical research. As institutions navigate these turbulent funding waters, they must remain vigilant in prioritizing ethical oversight to ensure that the commitment to patient-centered research remains strong.
Reinforcing Collaborative Efforts for Research Sustainability
To counterbalance the effects of funding cuts, reinforcing collaborative research efforts is essential for sustainable progress in medical research. By fostering partnerships among institutions, researchers can pool resources, share expertise, and streamline processes to ensure patient safety remains a top priority. Collaborative initiatives, such as multi-site studies, can enhance the quality of research while distributing the financial burden among participants. Such cooperation can lead to more efficient use of limited resources and bolster the overall impact of research endeavors.
In addition, collaborations can create new opportunities for funding through joint proposals, grants, and alternative revenue streams. Interdisciplinary partnerships that incorporate academia, industry, and community organizations can leverage diverse funding sources to sustain critical research projects. By coming together to support patient safety and ethical standards, the medical research community can navigate funding challenges more effectively and continue to advance the frontiers of science while upholding the dignity and rights of participants.
Looking Ahead: The Future of Medical Research Funding
As we look to the future of medical research funding, it is imperative to address the current challenges to ensure patient safety and ethical conduct of clinical trials. The funding landscape is constantly evolving, with potential opportunities for new funding models that prioritize patient welfare. Institutions need to explore innovative methods, such as public-private partnerships or crowdfunding for specific projects, that can fill existing gaps left by traditional funding sources. The goal must remain to foster an environment where ethical research can thrive and patients can trust in the processes that govern their care.
Furthermore, advocacy plays a crucial role in shaping the future of research funding. Engaging policymakers and stakeholders to highlight the importance of sustained investment in research can help secure the necessary resources for ongoing and future studies. As we grapple with the implications of current funding cuts, it is vital for the research community to unite in advocacy efforts that emphasize patient safety, ethical oversight, and the importance of collaborative research in driving meaningful advancements in healthcare.
Frequently Asked Questions
How do NIH funding cuts affect patient safety in medical research?
NIH funding cuts can severely impact patient safety in medical research by limiting resources available for Institutional Review Boards (IRBs) that oversee clinical trials. Without adequate funding, IRBs may struggle to effectively review research protocols, which is essential for ensuring the protection of participants’ rights and welfare. This disruption can lead to fewer safeguards against risks in research studies, ultimately jeopardizing patient safety.
What is the role of IRB oversight in medical research funding?
IRB oversight is crucial in medical research funding as it ensures that studies comply with ethical standards and participant protections. Each NIH-funded study involving human participants must undergo review by an IRB, which evaluates the research design, informed consent processes, and risk management strategies. Adequate medical research funding supports this oversight infrastructure, helping to maintain high ethical standards in clinical research.
How does funding cut impact clinical research ethics?
Funding cuts can compromise clinical research ethics by limiting support for the ethical oversight mechanisms like IRBs. This could result in inadequate review processes for research proposals, potentially exposing participants to ethical misconduct. Maintaining robust medical research funding is key to upholding clinical research ethics and ensuring that studies prioritize the well-being and rights of participants.
What are the consequences of funding cuts on the impact of research?
Cuts to medical research funding can substantially diminish the overall impact of research by slowing down project timelines, limiting the number of participating sites, and causing delays in new clinical studies. This can erode public trust in the research process and hinder the development of new treatments, ultimately affecting patient outcomes. Sustaining adequate funding is critical to maximizing the impact of medical research on public health.
Why is patient safety in research a priority in medical research funding?
Patient safety in research is a priority in medical research funding because it directly influences the ethical conduct of studies and the integrity of scientific findings. Funding supports the infrastructure necessary for IRB oversight, enabling thorough evaluation of research proposals. Safeguarding participants through adequate funding enhances trust in the medical research process and promotes responsible scientific advancement.
| Key Aspect | Details |
|---|---|
| Funding Freeze | Trump administration froze over $2 billion in federal research grants to Harvard, disrupting medical research oversight. |
| Impact on Patients | Halts in funding may compromise the rights and safety of patients participating in medical studies. |
| Role of IRBs | IRBs protect patients by reviewing research proposals, ensuring informed consent, and assessing risks. |
| Historical Context | Previous unethical experiments highlight the need for oversight and the legitimate concern for participant safety. |
| Consequences of Cuts | Funding cuts jeopardize research, risk participant safety, and hinder ethical oversight procedures. |
Summary
Medical research funding is critical for ensuring the safety and well-being of patients involved in clinical studies. The halt in federal funding has significant implications for both researchers and participants, as it disrupts the critical oversight provided by Institutional Review Boards (IRBs). Without adequate funding, the regulatory and ethical foundations of medical research could be compromised, leading to potential risks for participants and a decline in public trust in medical research initiatives. As highlighted in recent discussions, the integrity of human research depends heavily on robust oversight supported by consistent funding to ensure the safety of those involved.