TIM-3 therapy is an innovative approach that harnesses the body’s immune system to potentially address Alzheimer’s disease treatment. Recent studies have revealed that by inhibiting the TIM-3 protein, which serves as an immune checkpoint molecule, researchers can unleash microglia to target and eliminate harmful plaques accumulating in the brain. This breakthrough suggests a promising pathway for cognitive improvement, as evidenced by improved memory in mice subjected to this therapy. The findings not only spark hope in the realm of neurodegenerative diseases but also highlight the intriguing intersection between techniques used in cancer immunotherapy and Alzheimer’s treatment. As we delve deeper into these findings, the potential to transform traditional methods of managing Alzheimer’s disease becomes increasingly apparent.
The novel TIM-3 strategy represents a pivotal shift in treatment modalities for Alzheimer’s disease and aligns with advanced immunological approaches previously seen in cancer therapies. By blocking the inhibitory effects of the TIM-3 molecule, researchers have discovered a way to reactivate the brain’s immune cells, known as microglia, which are vital for clearing plaque and maintaining cognitive functions. This represents a significant advancement in understanding the role of immune checkpoint molecules in neurodegenerative disorders. As research progresses, leveraging this approach could redefine therapeutic standards for cognitive decline, with implications extending far beyond just cancer treatments. Exploring the transformative capabilities of TIM-3 therapy offers fresh insights into combatting the complex pathology of Alzheimer’s.
Exploring TIM-3 Therapy in Alzheimer’s Disease
Recent research has brought to light the potential of TIM-3 therapy as a novel treatment for Alzheimer’s disease (AD). TIM-3, an immune checkpoint molecule, inhibits microglial activity, the brain’s resident immune cells. By deleting the TIM-3 gene in mouse models, researchers observed enhanced microglial function, leading to the clearance of amyloid plaques associated with AD. This study marks a significant advancement in Alzheimer’s disease treatment, highlighting how manipulating immune responses can facilitate cognitive improvements by restoring memory functions in affected mice.
The exciting implications of TIM-3 therapy extend beyond basic research, presenting a promising avenue for clinical application in human AD patients. For instance, anti-TIM-3 antibodies may be utilized to block the inhibitory effects of TIM-3, allowing microglia to target and eliminate the harmful plaques. As our understanding of immune checkpoint molecules deepens, TIM-3 therapy could redefine therapeutic strategies for Alzheimer’s disease, capitalizing on the body’s own immune mechanisms to combat cognitive decline and restore memory.
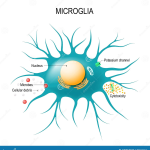
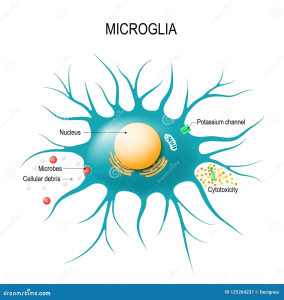
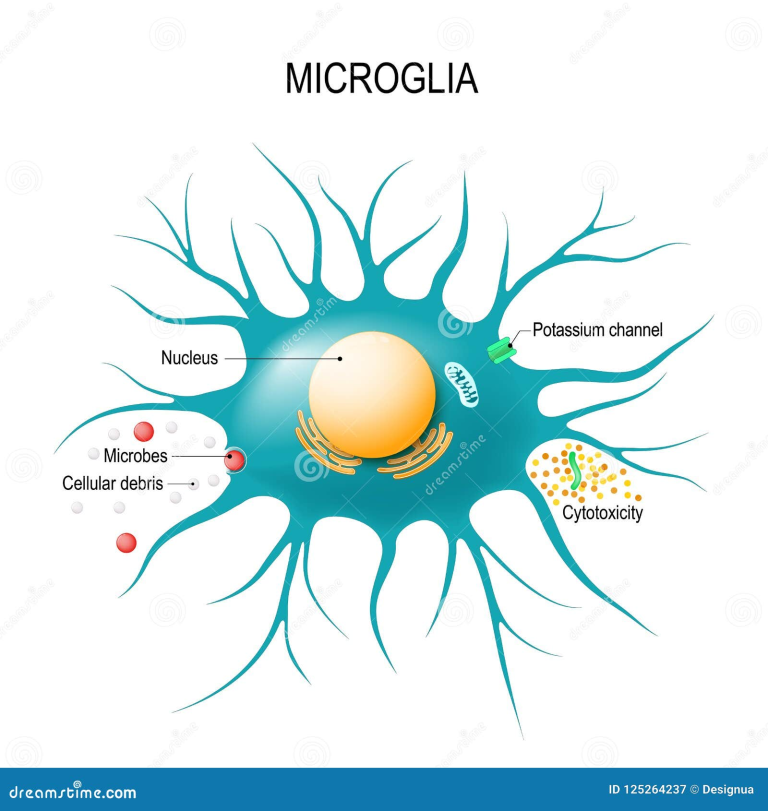
The Role of Microglia in Alzheimer’s Disease
Microglia play a critical role in maintaining brain health, particularly in the context of Alzheimer’s disease. As the brain’s immune cells, they are tasked with the removal of debris and plaques—functions essential for cognitive integrity. However, in AD, microglia become dysfunctional, often exacerbated by the presence of inhibitory molecules like TIM-3, which prevent them from attacking harmful amyloid plaques. This dysfunction underscores the necessity of harnessing microglial capabilities to fight Alzheimer’s effectively.
Recent findings indicate that alleviating the suppression of microglial activity not only helps in plaque clearance but also promotes cognitive improvements. When TIM-3 activity is inhibited, microglia regain their ability to phagocytize the amyloid-beta deposits, restoring synaptic function and aiding in memory retention. This transformative insight emphasizes the dual role of microglia—protectors that can also become hindered by the very mechanisms that are intended to regulate immune responses, posing challenges for Alzheimer’s disease treatment.
Checkpoint Molecules: A Double-Edged Sword
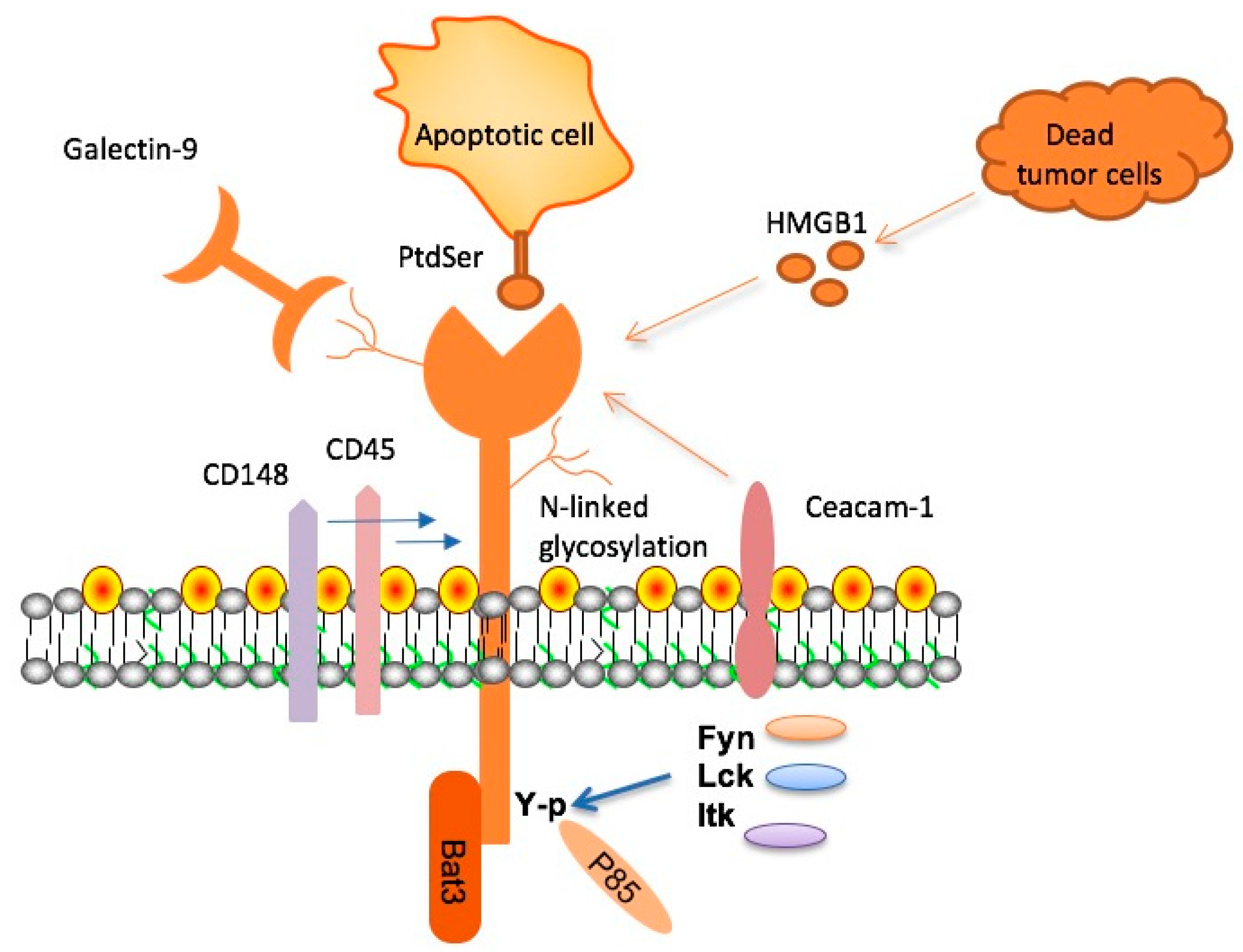
Checkpoint molecules, including TIM-3, are crucial for regulating immune responses in the body. While they protect against autoimmune reactions, their overexpression can lead to impaired immune function, exemplified in Alzheimer’s disease where microglia fail to clear amyloid plaques. Understanding this balance is essential, as these molecules can inadvertently inhibit protective immune responses, allowing neurodegenerative processes to progress unchecked.
The duality of checkpoint molecules positions them as potential targets for therapeutic intervention in Alzheimer’s disease. Research suggests that by inhibiting these molecules, such as through TIM-3 therapy, microglial efficacy can be restored, enabling them to combat the hallmark plaques of AD actively. Ongoing investigations into checkpoint inhibitors may reveal innovative strategies to enhance immune responses, potentially changing the trajectory of treatment for neurodegenerative diseases.
Furthermore, this perspective aligns with recent developments in cancer immunotherapy, where checkpoint inhibitors have revolutionized treatment strategies. The parallels between cancer and Alzheimer’s treatment illuminate the shared pathways and offer invaluable insights into how manipulating immune checkpoints can improve cognitive outcomes in patients suffering from Alzheimer’s disease.
Cognitive Improvement Through Immune modulation
The relationship between immune response modulation and cognitive improvement is a burgeoning field of research in Alzheimer’s treatment. By targeting molecules like TIM-3, scientists are uncovering pathways that could enable better cognitive function in AD patients. Studies utilizing animal models show promising outcomes where the inhibition of TIM-3 leads to enhanced memory and learning abilities, showcasing the transformative potential of immune-based therapies.
These findings point to a future where Alzheimer’s disease treatment is not solely focused on plaque removal but also includes strategies that enhance cognitive performance through immune system adjustments. As demonstrated with microglial activation in TIM-3-deficient mice, improved cognitive function is achievable. This shift towards immune modulation could pave the way for developing therapies that restore not only memory but also overall cognitive health, providing hope for millions affected by this debilitating disease.
Repurposing Cancer Therapies for Alzheimer’s Treatment
The innovative approach of repurposing existing cancer therapies, especially those targeting immune checkpoint molecules like TIM-3, for treating Alzheimer’s disease reflects a strategic advancement in neuroscience. With a wealth of experience from cancer immunotherapy, researchers are now investigating how the same modalities can be applied to neurodegenerative conditions. This cross-disciplinary approach is rich with potential, as existing anti-TIM-3 antibodies may be tailored to re-activate microglial clearance capabilities in AD.
The translational research effort highlights the potential of cancer therapeutics in addressing the complexities of Alzheimer’s disease. By bridging the gap between oncology and neurology, scientists aim to leverage successful mechanisms that stimulate immune responses to develop effective treatments tailored for cognitive restoration. With the increasing understanding of TIM-3’s role in both cancer and Alzheimer’s, a pathway emerges for therapies that offer renewed hope in combating cognitive decline.
Understanding Amyloid Plaques and Their Clearance
Amyloid plaques are a hallmark of Alzheimer’s disease, forming in the brains of affected individuals and contributing significantly to cognitive decline. The role of microglia in clearing these plaques is vital, as these cells are designed to maintain neural health by engulfing and digesting pathological debris. Unfortunately, in Alzheimer’s, microglial efficiency dramatically decreases, leading to plaque accumulation, neuroinflammation, and subsequent cognitive impairment.
Unlocking the mechanisms behind amyloid plaque clearance through TIM-3 inhibition shows promise for new treatment paradigms. By enhancing microglial activity, it may be possible not only to reduce plaque burden but to also ameliorate the neurodegenerative processes that underlie Alzheimer’s disease. This revelation opens doors for therapies that could significantly alter the disease’s progression and restore memory function, offering greater hope to those affected by its devastating effects.
The Future of Alzheimer’s Research and TIM-3 Therapy
As Alzheimer’s research continues to evolve, the focus on TIM-3 therapy signifies a critical pivot towards immune-based interventions. With the cumulative evidence supporting the role of TIM-3 as a therapeutic target, future studies are likely to concentrate on clinical applications and the effects of TIM-3 modulation in humans. Utilizing anti-TIM-3 antibodies in clinical trials could lead to groundbreaking advancements in Alzheimer’s disease treatment, shifting the paradigm away from traditional approaches.
Furthermore, the hope is that these developments will translate into effective interventions that improve the quality of life for patients. As the scientific community collaborates across disciplines, bringing together expertise from immunology, neurology, and beyond, the prospects for innovative therapies that effectively tackle Alzheimer’s disease appear increasingly promising.
Limitations and Challenges in Alzheimer’s Treatment
Despite the promising developments in TIM-3 therapy and immune modulation strategies, several challenges remain on the path to successful Alzheimer’s treatment. The complex nature of neurodegenerative diseases, characterized by multifaceted pathologies, necessitates comprehensive approaches that can address these intricacies. Additionally, translating findings from animal models to human applications introduces several variables that need thorough investigation.
The importance of understanding potential side effects or unintended consequences of altering immune responses cannot be understated. Researchers must navigate these complexities to ensure safety and efficacy in any new therapies targeting TIM-3. The ongoing exploration of immune checkpoint modulators will require careful balancing of therapeutic benefits against the risk of over-activating immune responses, emphasizing the need for rigorous clinical trials.
The Role of Genetic Factors in Alzheimer’s Disease
Genetic predisposition plays a significant role in the development of Alzheimer’s disease, with certain polymorphisms, such as those related to TIM-3, influencing individual susceptibility. The link between specific genes and late-onset Alzheimer’s disease highlights the importance of genetic research in understanding the condition’s pathology. Identifying genetic risk factors associated with TIM-3 can pave the way for advancements in targeted therapies that take into account an individual’s genetic makeup.
Additionally, recognizing the role of genetic factors may assist in stratifying patients for personalized treatment plans. Future therapies focusing on TIM-3 may be tailored to those with specific genetic profiles, optimizing therapeutic outcomes and improving treatment efficacy. As researchers delve deeper into the genetic underpinnings of Alzheimer’s, the potential for preventative strategies and targeted interventions becomes increasingly attainable.
Frequently Asked Questions
What is TIM-3 therapy and its potential for Alzheimer’s disease treatment?
TIM-3 therapy targets the TIM-3 molecule, an immune checkpoint that inhibits microglia, the brain’s immune cells, from clearing amyloid plaques associated with Alzheimer’s disease. By blocking TIM-3, this therapy could enhance the microglia’s ability to attack these plaques, potentially improving cognitive function and memory in Alzheimer’s patients.
How does TIM-3 affect microglia in Alzheimer’s disease?
In Alzheimer’s disease, microglia express high levels of TIM-3, which hampers their ability to remove plaque from the brain. TIM-3 acts as a checkpoint molecule that keeps microglia in a ‘resting’ state, preventing them from engaging and clearing out harmful plaques that accumulate and impair cognitive function.
Can TIM-3 therapy lead to cognitive improvements in Alzheimer’s patients?
Preliminary studies indicate that deleting the TIM-3 gene in mouse models of Alzheimer’s disease improves plaque clearance by microglia, leading to enhanced cognitive performance, suggesting that TIM-3 therapy could have similar effects in humans by restoring memory functions.
What is the role of immune checkpoint molecules like TIM-3 in Alzheimer’s disease?
Immune checkpoint molecules like TIM-3 regulate the immune response by preventing overactivation. In the context of Alzheimer’s, TIM-3 inhibits microglia from clearing amyloid plaques, which can lead to cognitive decline. Targeting TIM-3 might help reactivate these immune cells to clear harmful deposits in the brain.
How does TIM-3 therapy compare to traditional Alzheimer’s treatments?
Traditional Alzheimer’s treatments focus primarily on symptomatic relief, often with limited effectiveness. In contrast, TIM-3 therapy aims to enhance the brain’s immune response to actively clear plaques, potentially addressing the underlying pathology of Alzheimer’s disease rather than just alleviating symptoms.
What is being researched regarding the application of TIM-3 therapy in cancer immunotherapy?
TIM-3 is a recognized immune checkpoint molecule in cancer immunotherapy, where blocking TIM-3 can restore T cell function against tumors. The discovery of its role in Alzheimer’s disease opens up new avenues for using anti-TIM-3 antibodies to enhance immune responses in both conditions, showing how cancer research can inform Alzheimer’s treatment strategies.
What are the potential side effects of TIM-3 therapy in patients with Alzheimer’s disease?
While TIM-3 therapy has the potential to improve plaque clearance and cognitive function in Alzheimer’s patients, it is essential to monitor for side effects such as autoimmune responses or overactivation of the immune system. As with any therapy that modifies immune function, careful assessment of risks will be necessary during clinical trials.
What are the next steps for TIM-3 therapy in Alzheimer’s research?
Future research involving TIM-3 therapy includes testing anti-TIM-3 antibodies in mouse models engineered to express human TIM-3. This approach aims to evaluate the efficacy of TIM-3 blockade in halting plaque development and restoring cognitive abilities, paving the way for clinical studies in humans.
| Key Point | Details |
|---|---|
| Overview of TIM-3 therapy | Research indicates that TIM-3 therapy, successful in some cancers, may be applied to Alzheimer’s treatment. |
| Role of TIM-3 | TIM-3 is a checkpoint molecule that inhibits microglia from clearing amyloid plaques in the brain. |
| Microglia Function | Microglia are brain immune cells critical in removing plaques and maintaining memory functions. |
| Impact of TIM-3 Deletion | Deleting TIM-3 in mice enhances plaque clearance and improves cognition. |
| Therapy Development | Potential treatment involves using anti-TIM-3 antibodies to block its inhibitory function. |
| Current Research Status | Ongoing studies aim to test human anti-TIM-3 in Alzheimer’s mouse models. |
Summary
TIM-3 therapy represents a promising approach in the fight against Alzheimer’s disease. Research has shown that targeting the TIM-3 molecule can revitalize the brain’s immune system, allowing it to clear harmful plaques and improve cognitive functions. As scientists continue to explore and develop TIM-3 therapies, there is growing optimism that these treatments could potentially benefit those suffering from Alzheimer’s, offering a new avenue for improving memory and overall brain health.